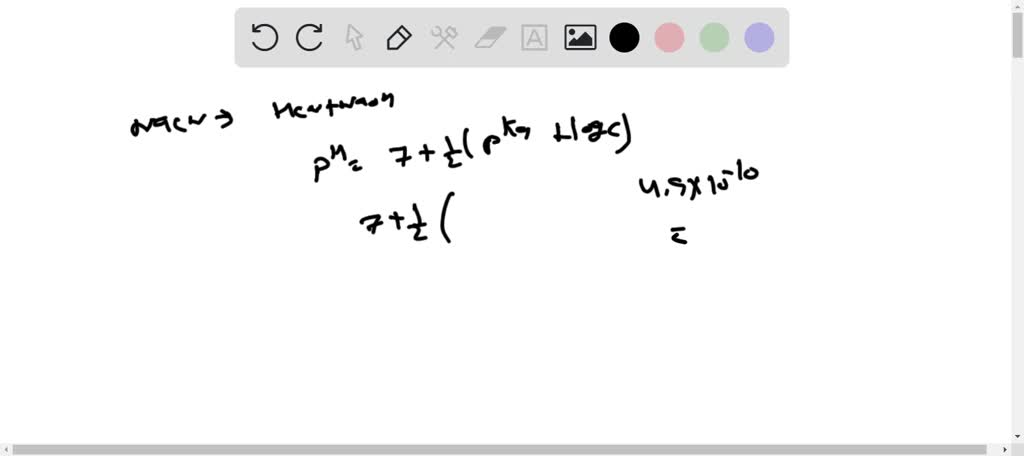
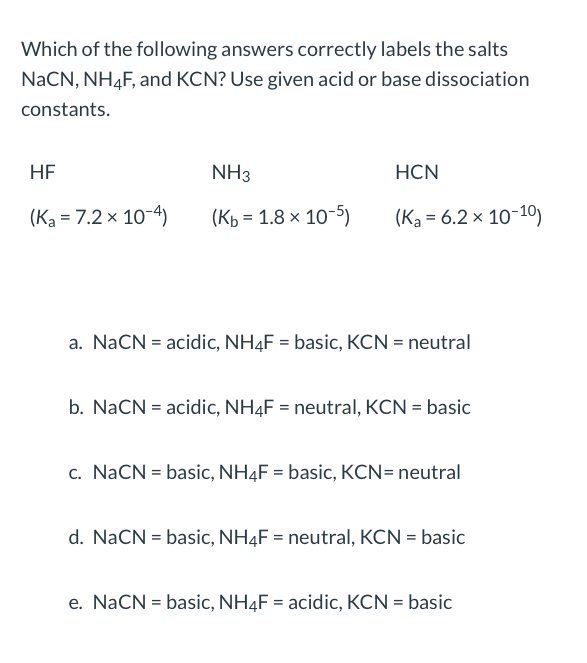
Exam 3 Practice Problems - Sample Problems (Acid/Base and Solubility) CHM Determine if the following - Studocu
✓ Solved: An unknown salt is either NaCN, NaC2H3O2, NaF, NaCl, or NaOCl. When 0.100 mole of the salt...
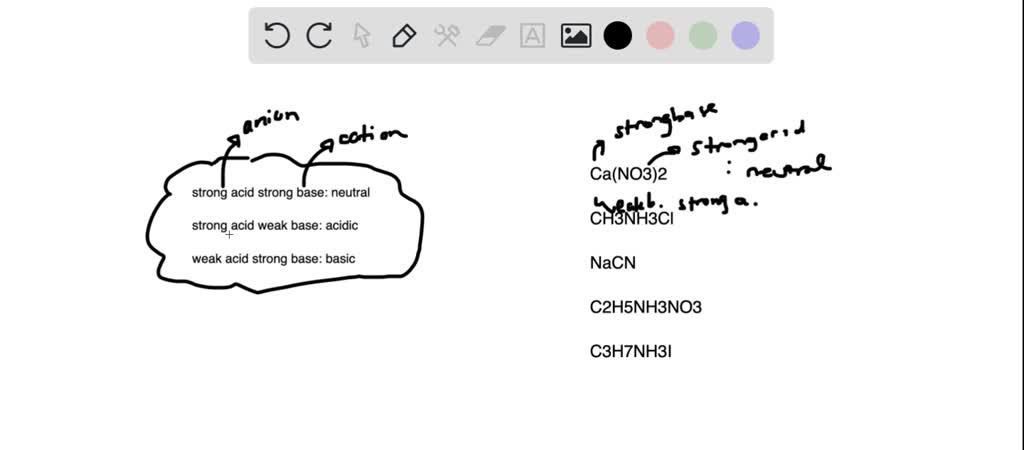
SOLVED: Consider 0.25 M solutions of the following salts. For each salt, indicate whether the solution is acidic, basic, or neutral. Ca(NO3)2 CH3NH3Cl NaCN C2H5NH3NO3 C3H7NH3I